Abstract
Introduction
Follicular lymphoma (FL) is the most common indolent lymphoma with a median survival approaching 20 years. However, there is significant clinical heterogeneity with a subset of patients experiencing transformation, early recurrence or refractory disease. Data from the National LymphoCare Study and others identified that progression of disease within 24 months of diagnosis in patients treated with chemoimmunotherapy (POD24) is associated with poor subsequent overall survival (OS). Using the Follicular Lymphoma Analysis of Surrogacy Hypothesis (FLASH) data, we sought to i) evaluate the association between FL international prognostic index (FLIPI) and other baseline factors on progression-free survival within 24 months of trial enrollment (PFS24) and ii) to validate POD24 as an early clinical endpoint in FL.
Methods
We performed a pooled analysis of 13 randomized clinical trials of patients in both the pre and post rituximab era (total sample size of 5,453, Table). Logistic regression model was used to evaluate the association between FLIPI, gender, and performance status (PS) with PFS24. Cox regression with POD24 as a time-dependent covariate was used to evaluate the association between POD24 and subsequent OS. An additional landmark analysis was conducted to evaluate the association of POD24 on OS for the subset of patients who were alive at 24 months post trial registration. Baseline Beta 2 Microglobulin (B2M) data were available for 9 of the 13 studies (total sample size of 4,361). Additional analyses including B2M along with other baseline factors were conducted on this subset of patients.
Results
29% of patients progressed and 2.5% died without progressive disease within 24 months from trial registration. Patients alive without progression at 24 months were younger and more commonly had favorable PS, limited stage, low FLIPI risk score, normal baseline hemoglobin, and normal baseline B2M. A multivariable logistic regression model indicated that being male (odds ratio (OR) = 1.35; 95%CI = (1.19 - 1.52); p < .01), having PS >= 2 (OR = 1.85; (1.47 - 2.38); p < .01) and high FLIPI risk score (3 - 5) (OR = 2.94; (2.38 - 3.57); p < .01 compared to low risk (0 or 1) were associated with increased risk of progression or death before 24 months. Elevated baseline B2M (>= 3mg/L) (OR = 1.47; (1.25 - 1.75); p < .01) was also associated with increased risk of progression or death before 24 months.
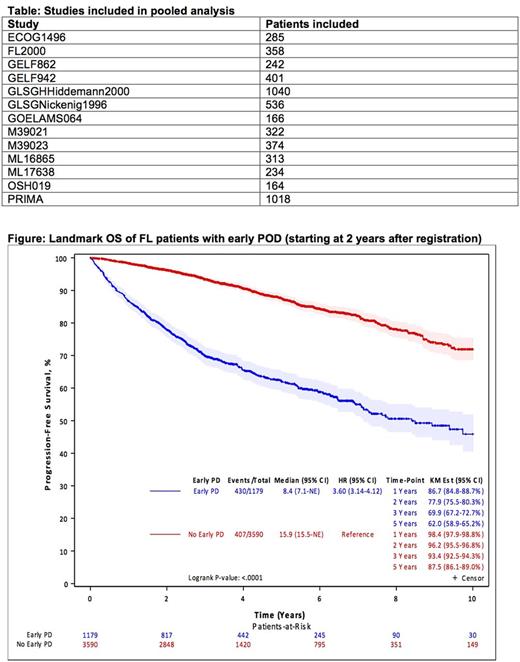
Results of the time-dependent Cox model, adjusted for gender and stratified by PS and FLIPI, indicate that POD24 was associated with poor subsequent OS (hazard ratio (HR) = 5.24; (4.63 - 5.93); p < .01). Results of the landmark analysis confirm the association between POD24 and OS in patients who were alive 24 months after trial registration (Figure). Similar results were observed in the subset of patients with baseline B2M after adjusting for its effect along with other covariates.
Conclusions
This pooled analysis of >5,000 patients with FL included in 13 prospective clinical trials is the largest cohort to date validating early progression as a robust indicator of poor FL survival. We identified male gender, poor PS, high FLIPI score, and elevated baseline B2M as predictors of early death and progression. These provide well-defined clinical factors for building comprehensive prognostic models in the future that incorporate clinical and molecular predictors of POD24. Results on maintenance and response to therapy as factors impacting POD24 will be updated in the presentation. Our results confirm POD24 as an early clinical endpoint of poor survival in FL that should be utilized to identify patients for prospective clinical trials.
Casulo: Celgene: Research Funding; Gilead: Honoraria, Other: travel support; Infinity: Consultancy. Salles: Janssen, Celgene, Novartis, and Amgen: Consultancy, Honoraria; Roche/Genentech: Consultancy, Honoraria, Research Funding; Gilead: Honoraria, Research Funding; Mundipharma: Honoraria. Hoster: Roche: Other: Travel support, Research Funding. Hiddemann: Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Marcus: Celgene: Other: Support for meeting attendance ; Roche: Consultancy, Honoraria, Other: Travel support, Speakers Bureau. Kimby: Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria; Abbvie: Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Pfizer: Research Funding. Vitolo: Mundipharma: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Nielsen: F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Foon: Celgene: Employment. Flowers: Burroughs Welcome Fund: Research Funding; Spectrum: Consultancy; Gilead: Consultancy; Onyx: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Research to Practice: Research Funding; Abbvie: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Janssen Pharmaceutical: Research Funding; Acerta: Research Funding; Infinity: Research Funding; Educational Concepts: Research Funding; Clinical Care Options: Research Funding; OptumRx: Consultancy; National Institutes Of Health: Research Funding; Seattle Genetics: Consultancy; V Foundation: Research Funding; Prime Oncology: Research Funding; Eastern Cooperative Oncology Group: Research Funding; National Cancer Institute: Research Funding; Genentech/Roche: Consultancy, Research Funding; Bayer: Consultancy; Millennium/Takeda: Research Funding; TG Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal